Physicochemical properties
Three SMAs of CH20, CH21 and CH22 might be efficiently synthesized based on our beforehand developed methodology21 with a superb yield in every step, whereas the artificial and characterization particulars have been offered within the Supplementary Strategies, Supplementary Figs. 1–25 and Supplementary Knowledge 1. Typically, a fascinating A-D-A structure might be indicated by plots of frontier orbital cost density variations (ΔQ) alongside molecular skeletons with a transparent peak-valley-peak form (Supplementary Fig. 26) for all three SMAs55. Notice that SMAs with such an A-D-A structure are anticipated to own improved gentle harvesting and cost technology/transport dynamics, decreased vitality losses and thus enlarged PCEs in ensuing OSCs in comparison with different sort molecules1,2,38,56,57. In good accordance with our discussions above, the isotropic polarizability of phenazine cores will increase progressively with the introduction of bromine, being 146.28 with out bromine, 166.26 with one bromine and 185.01 with two bromines (Fig. 1b). This tendency might be additionally discovered within the ensuing SMAs, displaying stepwise enlarged polarizability from CH20 to CH22 (Supplementary Fig. 27). As well as, the polarizability of dibrominated phenazine can be bigger than these of its difluorinated and dichlorinated analogs (Fig. 1b).
The most important dipole moments had been additionally noticed for each dibrominated phenazine and CH22 with respect to their counterparts (Supplementary Figs. 27 and 28). It’s price noting that CH22 contributes to a bigger relative dielectric fixed (εr) of three.32 than that of two.00 for CH20 and a couple of.35 for CH21 (Supplementary Fig. 29). This may very well be ascribed to each the enlarged molecular polarizability and dipole second of CH22 evaluating with these of CH20 and CH21. The elevated εr of natural semiconductors is conducive to realize facilitated cost switch/transport dynamics and thus offers rise to a greater OSC with amplified FF and JSC58,59. Moreover, based mostly on the differential scanning calorimetry (DSC) curves in Supplementary Fig. 30a, CH20, CH21 and CH22 exhibit exothermal peaks at 241.5, 253.9 and 286.8 °C, respectively, akin to a melting enthalpy (ΔHm) of 16.3 for CH20, 17.1 for CH21 and 23.3 J g−1 for CH22. The bigger ΔHm of CH22 than these of CH20 and CH21 suggests its stronger molecular interactions60, which shall be mentioned intimately under.
To guage the results of central unit brominating on light-harvesting capacities, the electron absorption spectra of SMAs had been recorded. As introduced in Fig. 1c, the utmost absorption peaks (λmax) of CH21 and CH22 dissolved in chloroform are at 733 and 729 nm, barely hypochromic shift by 7 and 11 nm, respectively, in comparison with that of 740 nm for CH20. Furthermore, CH21 and CH22 exhibit enlarged molar absorption coefficients of two.13 × 105 and a couple of.20 × 105 L mol−1 cm−1 compared to that of two.04 × 105 L mol−1 cm−1 for CH20, which could be ascribed to the suppressed spin prohibition of Q bands in CH21 and CH2250. An apparent red-shifting of λmax might be noticed from options to strong movies for all of the three SMAs (Fig. 1d), implying robust intermolecular interactions in strong states61,62. The HOMO and LUMO of CH20, CH21 and CH22 afforded by cyclic voltammetry (CV) measurements may very well be calculated as −5.61/−3.80 eV, −5.63/−3.80 eV and −5.67/−3.83 eV, respectively (Fig. 1e and Supplementary Fig. 31). Clearly, the progressively downshifted HOMOs needs to be ascribed to the introduction of electronegative bromine on central unit/donor of SMAs. In the meantime, the vitality gaps derived from CVs might be calculated as 1.36, 1.37 and 1.39 eV for CH20, CH21 and CH22, respectively. The progressively enlarged vitality gaps agree properly with the blue-shifted λonset as talked about above (Fig. 1d). Please be aware that the downshifted HOMO of CH22 will end in a comparatively bigger driving power for cost separation than CH20 and CH21 when the identical donor is utilized (Fig. 1f). The relative alignments of HOMO and LUMO agree properly the tendency rendered by density practical concept (DFT) calculations (see Supplementary Notice 1 and Supplementary Fig. 32).
As proven in Supplementary Fig. 30b, all three SMAs possess glorious thermal stability, indicated by a excessive decomposition temperature of greater than 330 °C. Furthermore, all three SMAs additionally exhibit glorious photostability, indicated by their UV-vis spectra. As proven in Supplementary Figs. 33 and 34, the form and depth of absorption spectra show virtually no modifications after ageing for over 550 h underneath one solar illumination63. Concurrently, the photostability of CH22 was additional confirmed by nuclear magnetic resonance (NMR) spectroscopy. There isn’t a change earlier than and after ageing for over 450 h underneath one solar illumination (Supplementary Fig. 35), which means that the introduction of C-Br bonds doesn’t lower the photostability of acceptors64. The superb thermal stability and photostability of those three SMAs can meet properly the chemical stability requirement as gentle absorption supplies. So as to make a transparent comparability, the associated physicochemical information of CH20, CH21 and CH22 have been summarized and introduced in Supplementary Tables 2 and 3.
Molecular packing in single crystals
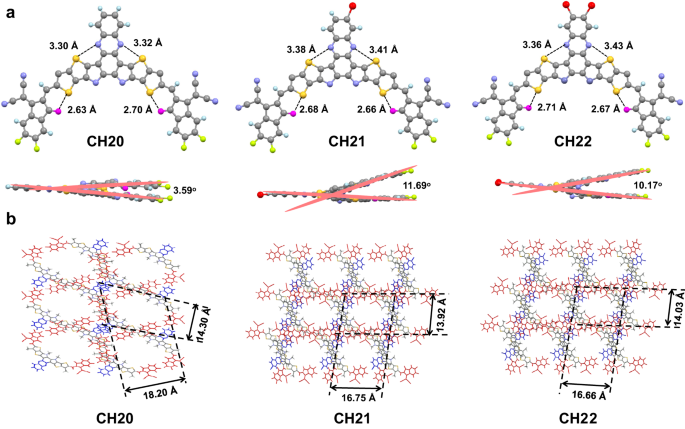
So as to unveil the results of central unit brominating on optimized molecular geometries and packing behaviors, single-crystal X-ray diffraction measurements of CH20, CH21 and CH22 had been carried out. By use of a sluggish solvent diffusion methodology, three single crystals featured with a lovely metallic luster had been afforded (see Supplementary Notice 2). Their detailed X-ray parameters and checked information concerning the construction elements and structural output of the three single crystals might be present in Supplementary Desk 4, Supplementary Figs. 36–38 and Supplementary Knowledge 2–4. As introduced in Fig. 2a, the hexyl decyl substitutions on SMAs had been omitted for clear remark. All three SMAs exhibit an identical banana-curved and helical molecular geometry. Notice that the distances of S…N between phenazine and bridged thiophene are ~3.3–3.4 Å for all of the three acceptors, barely smaller than the sum van der Waals radii (~3.55 Å when utilizing the values of Alvarez65) of S and N, indicating the potential noncovalent interactions between S and N in these SMAs. This is also supported by the decreased density gradient (RDG) evaluation indicated by the sunshine blue iso-surfaces between S and N atoms (Supplementary Fig. 39)66,67,68. Notice that the efficient noncovalent S…O = C and S…N secondary interactions additional assure the comparatively planar conjugated backbones21,29,32. Amongst them, CH21 and CH22 exhibit a barely distorted skeleton with a bigger dihedral angle of ~11° between two finish teams than that of three.59° for CH20, which needs to be attributable to the bromine-induced steric hindrance on central items. From the general view in Fig. 2b, all three crystals might be categorised as triclinic techniques, and extra importantly, the favorable three-dimensional (3D) molecular packing networks might be properly established. As well as, CH21 and CH22 possess rectangle-shaped voids of ~16.75 × 13.92 Å and ~16.66 × 14.03 Å, respectively, smaller than that of ~18.20 × 14.30 Å for CH20 with a rectangle-shaped void. It’s price mentioning that the favorable 3D molecular packing networks are normally constructed by each central and finish items in CH-series SMAs and have been confirmed to be conducive to get superior cost separation/transport/recombination dynamics in OSCs28.
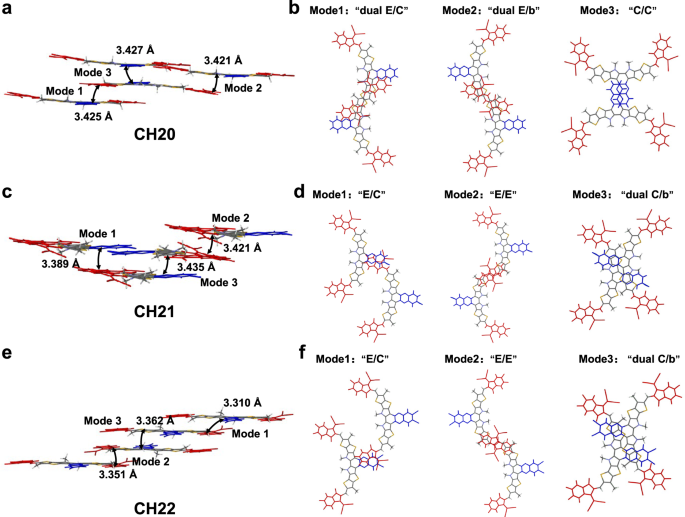
The various 3D molecular packing networks for CH20, CH21 and CH22 needs to be on the idea of various intermolecular packing modes, which may very well be dramatically affected by even a little or no structural modification (like totally different halogenations) on the central unit of CH-series SMAs29. Due to this fact, all of the intermolecular packing modes with intermolecular potential over 70 kJ mol−1 had been extracted from single crystals of CH20, CH21 and CH22 (Fig. 3), and an in depth evaluation was introduced in Desk 1. As regards CH20 with out bromine on the central unit, three packing modes might be noticed, that are assigned to twin finish to central items (“twin E/C” mode), twin finish to bridge items (“twin E/b” mode) and central to central items (“C/C” mode). Amongst them, the “twin E/C” mode with a small π…π stacking distance of three.425 Å and an intermolecular potential of 190.5 kJ mol−1 was solely noticed in CH-series SMAs to date and performed a crucially vital position in establishing superior 3D packing networks21,29. Extra apparently, the traditional packing mode of end-to-end items (“E/E” mode), which exists broadly and even performs a extremely vital position in high-performance ITIC and Y-series SMAs1,54,61,69,70, vanishes from sight within the crystal of CH20 together with the emergence of a never-observed but additionally efficient “C/C” mode. The potential purpose for forming “C/C” packing mode in CH20 could also be that the thermodynamically secure state of “C/C” packing is extra preferential through the very sluggish crystallization course of as a result of apparent π-conjugated growth of the central unit. Nonetheless, with brominating on the central unit of CH20, the “C/C” packing mode disappears, and the extremely efficient “E/E” packing mode reemerges once more with a big intermolecular potential of ~129 kJ mol−1. This needs to be ascribed to the comparatively giant steric hindrance of bromine on central items of CH21 and CH22, which considerably weakens the tendency for forming “C/C” packing mode and facilitates the formation of “E/E” packing in flip. In addition to, one other two distinctive packing modes of end-to-central items (“E/C” mode) and twin central to bridge items (“twin C/b” mode) may very well be additionally noticed in crystals of CH21 and CH22. Specializing in these three packing modes in CH20, CH21 and CH22, dibrominated CH22 reveals a smaller common π⋯π distance (“E/C” mode: 3.310 Å, “E/E” mode: 3.351 Å, “twin C/b” mode: 3.362 Å) than these of CH20 (“twin E/C” mode: 3.425 Å, “twin E/b” mode: 3.427 Å, “C/C” mode: 3.421 Å) and CH21 (“E/C” mode: 3.389 Å, “E/E” mode: 3.421 Å, “twin C/b” mode: 3.435 Å). This will likely profit from the simpler Br⋯S, Br⋯π or halogen bonding interactions41,49 within the crystal of CH22 regardless of the comparatively giant steric hindrance of bromine. Notice that the favorable molecular packings endow with CH22-based neat movie an improved electron mobility (μe, Supplementary Fig. 40a, b) of 4.63 × 10−4 cm2 V−1 s−1, in comparison with that of CH20 (2.14 × 10−4 cm2 V−1 s−1) and CH21 (3.45 × 10−4 cm2 V−1 s−1)21,29,70.
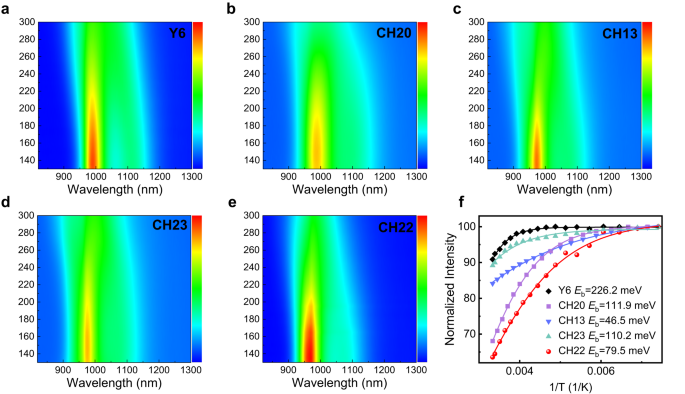
Additionally, in an effort to unveil the change tendency of exciton binding vitality (Eb), we took a number of single-crystal constructions of CH-series SMAs to theoretical calculations and made a comparability with typical Y6 within the gasoline section (Supplementary Fig. 41 and Supplementary Desk 5). In consequence, the Eb of CH-series SMAs is way decrease than that of Y6 and, extra importantly, additionally decreases with halogenation on central items, akin to from Y6 to CH20 and CH22. It’s well-known that Eb is influenced by not solely molecular constructions but additionally solid-state polarization results and intermolecular digital interactions34,71. Due to this fact, the calculated values of Eb proven in Supplementary Desk 5 are dramatically bigger than that noticed within the strong section based mostly on the temperature-varying photoluminescence (PL) spectra72. As displayed in Fig. 4, all of the CH-series SMAs possess a enormously smaller experimental Eb (~100 meV) than that of 226 meV for the Y6 movie, indicating some great benefits of expanded conjugation of central items. Extra importantly, Eb may lower with halogenation on the central unit of CH-series SMAs, making it excessive potential for attaining a a lot smaller Eb (even akin to inorganic semiconductors) if additional halogenation on the central unit is carried out to delicately tune each molecular constructions and intermolecular packing modes.
To sum up, CH20 with a non-brominated central unit was noticed with a robust intermolecular packing mode of “C/C” as a result of nice π-conjugated extension of the central unit, thus suppressing the formation of the traditional packing mode of “E/E”. After brominating on the central unit, the “C/C” packing mode disappears, and the extremely efficient “E/E” packing mode reemerges, indicating that the brominating on the central unit of SMAs has a profound impact on intermolecular packing behaviors. Extra importantly, the “E/E” packing mode has been confirmed to play a really essential position in establishing the favorable 3D molecular packing networks of SMAs and thus results in extra environment friendly cost transport. Due to this fact, on such a superb molecular platform of CH-series SMAs, brominating on a central unit fairly than an finish unit may higher stability the totally different intermolecular packing modes and afford a a lot smaller Eb, thus maximizing some great benefits of bromination (like excessive crystallinity, environment friendly halogen bonding, and so forth.) whereas circumventing its opposed results on molecular packings.
Photovoltaic performances
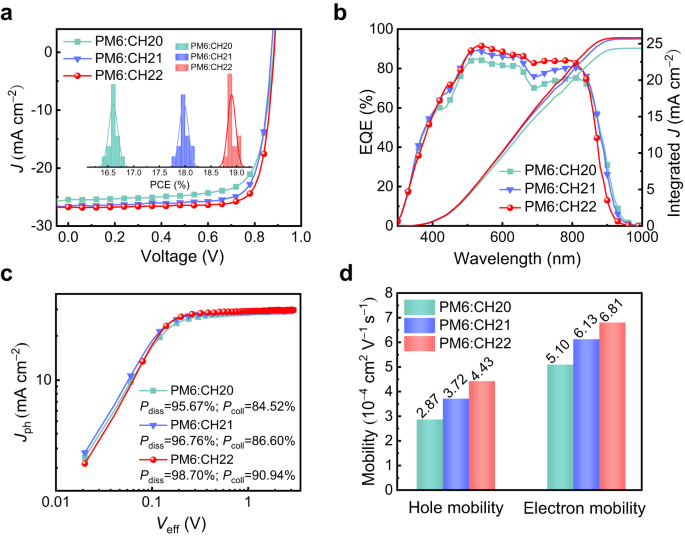
The various intermolecular packing modes of those SMAs ought to result in a lot totally different photovoltaic performances of ensuing OSCs in concept. Thus, OSCs featured with a tool structure of ITO/PEDOT:PSS/energetic layer/PNDIT-F3N/Ag had been fabricated, during which a polymeric donor PM64 was blended with CH20, CH21 and CH22 to compose energetic layers due to its matched vitality ranges and absorptions (Supplementary Fig. 42). Detailed machine fabrication and characterization procedures had been offered in Supplementary Data (Supplementary Tables 6–8). The present density-voltage (J–V) traits of the optimum OSCs are proven in Fig. 5a and illustrated in Desk 2. For CH20-based binary OSCs, a PCE of 16.79% with a VOC of 0.881 V, a JSC of 25.44 mA cm−2 and an FF of 74.92% is delivered. Benefiting from the optimized molecular packings tuned by brominating on central items, a enormously improved PCE of 18.12% is yielded by a CH21-based machine accompanied by a VOC of 0.873 V, a JSC of 26.57 mA cm−2 and an FF of 78.13%. Extra excitingly, a champion PCE of 19.06% is additional generated by CH22-based OSCs featured with a VOC of 0.884 V, a JSC of 26.74 mA cm–2 and an impressive FF of 80.62%, which has been ranked among the many first-class OSCs to date10,11 and likewise reached the perfect PCE for CH-series SMAs-based OSCs21,28. The statistic effectivity evaluation for 15 impartial OSCs (see the detailed machine parameters in Supplementary Tables 9–11) was inset in Fig. 5a, and the common efficiency from CH20 to CH22 additionally reveals an enlarged tendency. The corresponding exterior quantum effectivity (EQE) plots of CH20-, CH21- and CH22-based units had been displayed in Fig. 5b. Notice that the built-in present densities derived from EQE plots are 24.69, 25.99, and 26.17 mA cm−2, respectively, which matched properly with these afforded by J-V exams (inside 3% error). Though the progressively blue-shifted absorption from CH20 to CH22 renders a stepwise shrunken EQEs spectra, the general greater EQE responses within the vary of 450–850 nm for CH22 contribute to the biggest built-in present densities. Notice that the various EQE values are concerned with a number of elements, such because the efficiencies of sunshine harvest, exciton dissociation, cost transport, and so forth. Herein, the simpler exciton dissociation attributable to a comparatively giant driving power (Fig. 1f) and facilitated cost transport induced by superior nanoscale movie morphologies (see the detailed discussions under) for CH22 ought to account for its finest EQEs and built-in present density. As well as, the considerably improved FF for CH22-based OSCs needs to be attributable to facilitated cost transport. It’s also price noting that PCEs of PM6:CH22-based OSCs may very well be maintained above 96% and ~85% in comparison with its preliminary PCEs after 1500 h underneath room temperature and 400 h underneath warmth therapy at 65 °C (Supplementary Fig. 43), respectively. The nice storage and thermal stabilities of PM6:CH22 system spotlight its potential for commercialization in large-scale manufacturing of high-performance OSCs.
a Present density-voltage curves of PM6:CH20, PM6:CH21 and PM6:CH22-based OSCs; the inset reveals a statistical evaluation of machine effectivity. b The EQE plots and built-in JSC curves. c Jph versus Veff curves of PM6:CH20, PM6:CH21 and PM6:CH22-based OSCs. d Histograms of the opening and electron mobility of blended movies.
So as to confirm the distinctive benefits of brominating on the central unit fairly than finish teams, as we now have mentioned above, we additional constructed the SMAs of CH22-6Br with di-bromination on each central unit and finish teams. As proven in Supplementary Fig. 44 and Supplementary Desk 12, CH22-6Br solely achieved a PCE of 17.00% with considerably decreased JSC and FF in comparison with these of CH22, which can be attributable to not solely the extreme molecular aggregation but additionally the much less compact π-π stacking of molecules as a result of comparatively bigger steric hindrance of bromine than its homomorphic fluorine or chlorine. The extreme molecular aggregation of CH22-6Br might be confirmed by the comparatively bigger fiber measurement (12.7 nm for PM6:CH22-6Br, 11.9 nm for PM6:CH22) based mostly on a statistical evaluation of atomic power microscopy (AFM) section photographs (Supplementary Fig. 45) and barely larger crystal coherence size (CCL) of 23.37 Å in CH22-6Br neat movie estimated from GIWAXS (Supplementary Fig. 46 and Supplementary Desk 13) evaluating to that of CH22 (22.53 Å). Furthermore, the marginally bigger π-π stacking distances for CH22-6Br (3.74 and three.70 Å for neat and blended movies) might be estimated from GIWAXS in comparison with that of CH22 (3.66 and three.63 Å for neat and blended movies), which can be attributable to the comparatively bigger steric hindrance of bromine.
To additional perceive the basic causes for JSC and FF enlargement in CH21- and CH22-based units with respect to that of CH20, each the efficiencies for exciton dissociation (Pdiss) and cost assortment (Pcoll) had been first investigated (Fig. 5c). In contrast with that of 95.67%/84.52% for CH20-based machine, the values of Pdiss/Pcoll might be estimated as 96.76%/86.60% and 98.70%/90.94% for CH21- and CH22-based units, respectively. The improved Pdiss needs to be ascribed to the downshifted HOMO vitality ranges of CH21 and CH22, which is able to theoretically result in a bigger driving power for exciton splitting9,34. As well as, the enlarged Pcoll for CH21 and CH22-based units needs to be attributed to superior cost transportation dynamics35. This may be manifested by the smaller cost extraction time of 0.32 μs for CH22 and 0.39 μs for CH21-based units in comparison with that of 0.48 μs for CH20-based one (see the transient photocurrent decay curves in Supplementary Fig. 47). As we now have mentioned above, the larger polarizability and stronger delocalization impact with the introduction of bromine on SMAs are anticipated to end in stronger intermolecular interactions and elevated cost mobility. Due to this fact, the electron/gap mobilities (μe/μh) of PM6:CH20-, PM6:CH21- and PM6:CH22-based units had been evaluated by using the space-charge restricted present (SCLC) methodology, which might be decided to be 5.10/2.87, 6.13/3.72 and 6.81/4.43 × 10−4 cm2 V−1 s−1 akin to μe/μh ratios of 1.78, 1.65 and 1.54, respectively (Fig. 5d and Supplementary Fig. 40c, d). Such outcomes point out that the CH22 system with the strongest polarizability and largest dipole second amongst these three SMAs may successfully optimize the cost separation and transport dynamics to additional enhance JSC and FF in corresponding units.
Morphology evaluation
Because it has been broadly mentioned, the morphology of blended movies in OSCs performs even a dominant position in cost transport conduct and photovoltaic efficiency11,31. Due to this fact, we applied the AFM and transmission electron microscopy (TEM) measurements (Supplementary Figs. 48 and 49) to unveil the nanoscale morphology of blended movies. As proven in Supplementary Fig. 48a, all of the blended movies possess a uniform and easy floor. The values of root-mean-square (RMS) roughness of PM6:CH20 (1.02 nm), PM6:CH21 (1.13 nm) and PM6:CH22 (1.22 nm) elevated with the gradual introduction of bromine on the central unit as a result of enhanced molecular crystallinity from CH20 to CH22. In view of the section photographs (Supplementary Fig. 48b), fiber-like domains with correct sizes had been noticed, which has been confirmed to successfully facilitate cost transport in OSCs19. Based mostly on a statistical evaluation of nanofiber measurement in Supplementary Fig. 48c, d, a barely however progressively elevated fiber measurement might be noticed with 10.7 for CH20, 11.5 for CH21 and 11.9 nm for CH22, suggesting that brominating on central items may improve molecular crystallinity and fine-tune section area sizes. In the meantime, TEM photographs (Supplementary Fig. 49) additional corroborate the fibrillar community construction in all blended movies, which is conducive to attaining excessive cost mobility and avail cost transport. Notice that the nanofiber measurement is expounded to the miscibility between PM6 and SMAs; thereby, contact angles and derived Flory-Huggins interplay parameters (χ)73,74 for the corresponding donor and acceptor had been additional evaluated. As proven in Supplementary Fig. 50 and Supplementary Desk 14, χD:A for PM6:CH22 (0.74) and PM6:CH21 (0.63) are bigger than that of PM6:CH20 (0.46), indicating decrease D/A miscibility after brominating on the central unit of SMAs13. This will likely contribute to the upper area purity and bigger nanofiber measurement, which is in good accordance with outcomes from AFM and TEM photographs.
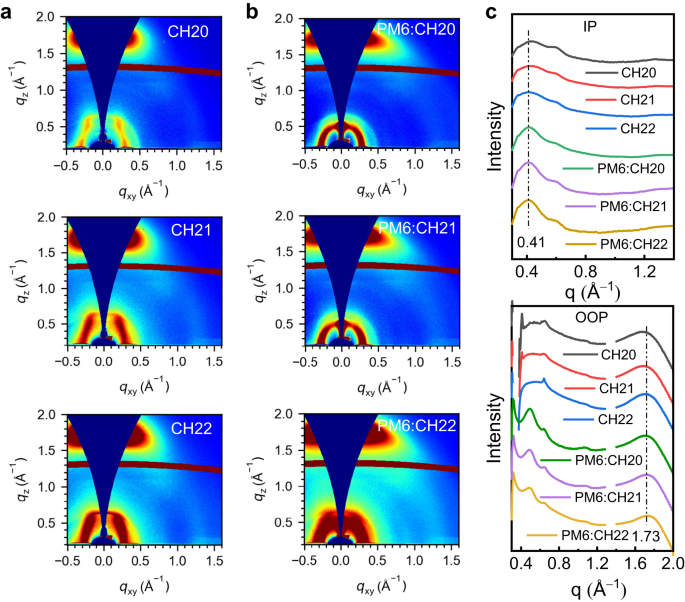
Then, the impact of brominating on a central unit of SMAs on molecular packing and orientation in neat and blended movies was studied by using GIWAXS. The 2D-GIWAXS patterns and line-cut profiles of CH20, CH21 and CH22 neat movies are introduced in Fig. 6, and corresponding parameters are summarized in Supplementary Desk 15. All of the neat movies for CH20, CH21 and CH22 exhibit pronounced (010) diffraction peaks situated at 1.663, 1.690 and 1.718 Å−1 in out-of-plane (OOP) route and sharp (100) diffraction peaks at 0.423, 0.418, 0.410 Å−1 in in-plane (IP) route, respectively, indicating the preferential face-on orientations. Notice that CH22 shows the extra outstanding (010) diffraction peaks with barely shifted to greater q area, assigned to a shorter π-π stacking distance of three.66 Å than these of three.78 Å for CH20 and three.72 Å for CH21, which is in good accordance with the evaluation of single crystals (Supplementary Fig. 51). In the meantime, a comparable however barely bigger CCL of twenty-two.53 Å might be noticed for CH22 neat movie evaluating to these of 21.10 Å for CH20 and 21.18 Å for CH21, indicating the improved molecular packing ordering. After mixing with the PM6 donor, the sharp (010) diffraction peaks and (100) diffraction peaks can nonetheless be noticed in OOP and IP instructions, respectively, suggesting that the fascinating face-on orientation may very well be properly maintained. As it’s summarized in Supplementary Desk 15, the (010) diffraction peaks within the OOP route for 3 blended movies are situated at 1.713, 1.718 and 1.733 Å−1, assigned to the π-π stacking distances of three.67, 3.66 and three.63 Å, respectively. In the meantime, CCLs of (010) diffraction peaks had been estimated to be 22.00, 22.09 and 22.44 Å for PM6:CH20, PM6:CH21 and PM6:CH22 blended movies, respectively. Typically, the extra compact π-π stacking and barely enhanced molecular packing order for CH22 movies needs to be contributed to the improved molecular crystallinity and response for the superior cost transport behaviors and even enlarged JSC and FF values in CH22-based OSCs75. Furthermore, the Urbach vitality (Eu) values had been calculated to be 20.7, 20.1 and 19.3 meV for CH20-, CH21- and CH22-based units, respectively, by becoming the Fourier rework photocurrent spectrometer EQE (FTPS-EQE) (Supplementary Fig. 52). The smaller Eu of PM6:CH22 additionally suggests the extra ordered molecular packing, which agrees properly with its barely bigger CCL76. All the outcomes above manifested that the delicately brominating on central items of SMAs may give rise to enhanced molecular crystallinity, extra compact intermolecular packings and crystalline orders, thus rendering extra environment friendly cost transport behaviors and higher photovoltaic efficiency.
Vitality loss evaluation
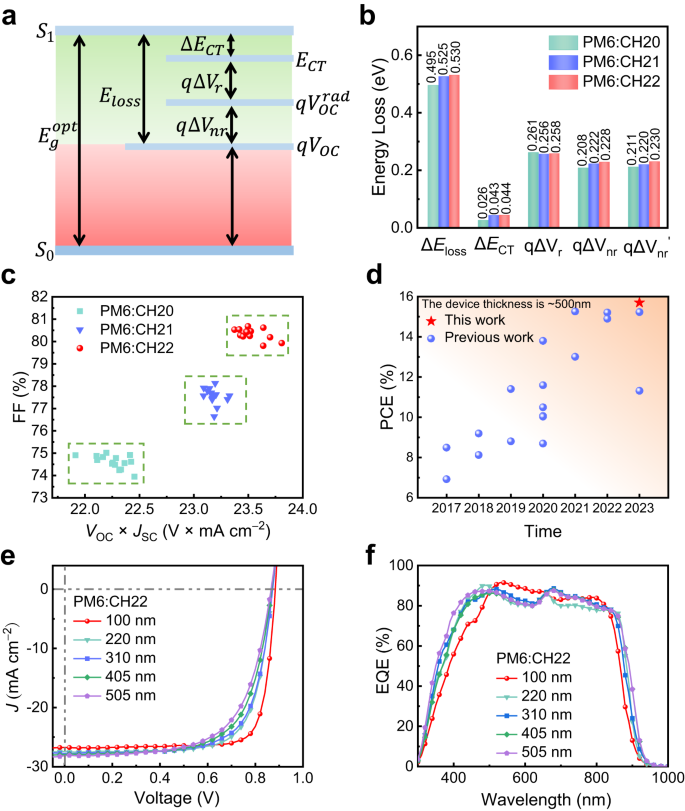
To review the affect of central unit brominating on vitality loss (Eloss) of SMAs, all three SMAs units had been ready. The entire Eloss for PM6:CH20, PM6:CH21 and PM6:CH22 techniques are 0.495, 0.525 and 0.530 eV, respectively (see Supplementary Notice 3 and Supplementary Figs. 53 and 54 for the detailed calculations). Notice that CH21 and CH22 techniques with brominating on central items afford the marginally bigger Eloss (Fig. 7, Supplementary Desk 16), particularly non-radiative recombination loss ((Delta {V}_{{nr}})). By becoming the low-energy area of extremely delicate EQE and EL spectra (Supplementary Fig. 54), the vitality degree (({E}_{{CT}})) of cost switch (CT) state might be decided to be 1.35, 1.35 and 1.37 eV for PM6:CH20, PM6:CH21, PM6:CH22 blended movies, respectively, giving rise to vitality offsets ((Delta {E}_{{CT}})) between native exciton state (LE) and CT state of 0.026, 0.043 and 0.044 eV. With such a CT state vitality close to to that of LE, the extremely potential hybridization of LE and CT states needs to be considered77,78. Notice that the smaller (Delta {E}_{{CT}}) for CH20 than these of CH21 and CH22 might end in simpler hybridization of LE and CT states, thus enhancing the luminescence of the CT state by way of the “depth borrowing” mechanism29,78 and additional suppressing the non-radiative recombination charge of CT states. This can be the rationale that CH20-based OSC reveals a decreased non-radiative recombination loss in comparison with that of CH21 and CH22-based ones. As well as, the comparatively bigger electron reorganization energies of 147.23 for CH21 and 148.87 for CH22 than that of 144.59 meV for CH20 might be noticed (Supplementary Fig. 55), which needs to be attributable to the simply polarizing property of bromine and likewise partially account for the bigger Eloss for CH21 and CH22-based OSCs. That is vital as it’s well-known {that a} small vitality loss needs to be rationally pursued on the premise of environment friendly cost switch/transport dynamics. Due to this fact, a well-balanced trade-off between JSC and VOC is very fascinating, particularly within the state-of-the-art OSCs. As displayed in Fig. 7c, the CH22-based machine achieved essentially the most compromise between tangle JSC and VOC regardless of its comparatively bigger vitality loss with respect to that of CH20, thus rendering the perfect FF to achieve the optimum effectivity.
a A schematic diagram of Eloss-related parameters. b Statistical diagram of detailed Eloss of CH20, CH21 and CH22-based units. c VOC×JSC vs FF of CH20, CH21 and CH22-based 15 independently measured OSCs. d A abstract of the PCE for thick-film OSCs with ~500 nm. e Present density-voltage curves of PM6:CH22-based OSCs with totally different thicknesses (100 nm, 220 nm, 310 nm, 405 nm, and 505 nm). f The corresponding EQE spectra.
Thick-film machine efficiency
As everyone knows, the overwhelming majority of high-performance OSCs are obtained with a layer thickness of ~100 nm and PCEs will lower drastically with growing thickness of energetic layers, which enormously limits their software in giant space printing course of utilizing essentially the most promising expertise of roll to roll79. Thus, it is extremely vital to develop high-performance OSCs with good tolerance of energetic layer thickness. It’s well-known that light-harvesting supplies with excessive crystallinity and mobility are useful to acquire higher efficiency with thick movies80. Due to this fact, CH22 is anticipated to realize a passable efficiency in thick-film units in gentle of its compact molecular packings and excessive crystallinity. Bearing these ideas in thoughts, PM6:CH22 units with totally different energetic layer thicknesses (~200, ~300, ~400 and ~500 nm) had been fabricated. The obtained J–V traits and EQE plots had been introduced in Fig. 7e, f, and the detailed parameters are listed in Supplementary Desk 17. With movie thickness growing, the JSC of CH22-based OSCs improves progressively, however FF declines enormously. In consequence, an eventual PCE of 15.70% is afforded by making use of a ~500 nm movie thickness of CH22-based OSC, demonstrating the very best PCE for OSCs with energetic layer thickness as much as 500 nm (Supplementary Desk 18). Notice that the enormously decreased FF could also be attributable to the extra trap-assisted recombination in thicker-film units, instructed by elevated S/(kT/q) values from 1.08 to 1.26 and decreased Pcoll from 90.94 to 80.68% for OSCs with a movie thickness from 100 to 500 nm (Supplementary Fig. 56).