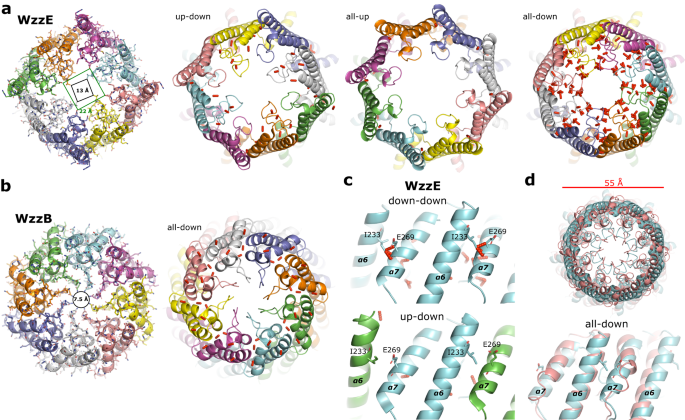
WzzE is a C4 symmetric octamer with alternating preparations of its L4 loops
We purified (Supplementary Fig. 1) and decided the construction of the absolutely intact polysaccharide co-polymerase WzzE from E. coli by cryo-electron microscopy to an general decision of three.2 Å. Per beforehand decided buildings of this household, an octameric bell-shaped complicated was recognized by reference-free ab-initio 3D classification and is clearly seen within the un-symmetrized (C1) map (Fig. 1a and Supplementary Fig. 2). An general decision of three.4 Å was obtained with out the applying of symmetry and, though clearly octameric in nature, displayed a lack of octameric symmetry within the area of the L4 loops on the prime of the periplasmic bell (Fig. 1a and Supplementary Fig. 2). On this area the L4 loops are seen in two conformations, one up and one down with, remarkably, the subunits clearly alternating between the up and down conformations across the octameric complicated. Though the applying of C8 symmetry did marginally enhance the general decision of the map to three.1 Å, the alternating nature of the L4 area was averaged out (Fig. 1a and Supplementary Fig. 3). Thus, as a way to resolve this alternating association of loops, C4 symmetry was utilized through the use of two adjoining subunits encompassing the 2 L4 conformations because the uneven unit leading to a 3.2 Å map. The top quality of the C4 symmetrized map allowed the whole amino acid sequence of the periplasmic area to be constructed (Fig. 1b and Desk 1), together with the dynamic L4 loops in alternating conformations.
a Ultimate reconstructed volumes of the full-length WzzE refined with out and with the applying of C4 and C8 symmetry. High: view wanting down from the highest of the periplasmic area. The appliance of C8 symmetry averages out the density for the alternating conformations of the L4 loops. Blue define represents the 4 uneven models. b Cartoon illustration of the octameric complicated overlaid with the C4 symmetrized WzzE map (white).
Total, the periplasmic area is similar to the beforehand described buildings7,8,10: a bell-shaped area ~100 Å in peak above the membrane interface that incorporates a hole inside. The bell-shaped complicated outcomes from a side-by-side packing of protomers and the interactions noticed listed below are additionally much like what have been described elsewhere7,8,10. This construction, nonetheless, incorporates alternating L4 loops situated on the prime of the periplasmic bell (residues 238–256). Structural alignments of two adjoining WzzE subunits to a single monomer of WzzB7 (Fig. 2a, b) reveal extremely comparable buildings on the single subunit degree regardless of low id of the amino acid sequence4 (Supplementary Fig. 4) with the place of the L4 loop being the principle distinction. The downward dealing with L4 loop of WzzE factors into the middle of the periplasmic bell and resembles the place seen in WzzB, though barely extra compact. The upward dealing with L4 loop alternatively, is flipped upwards and is at considerably decrease decision (Fig. 2 and Supplementary Fig. 3). As a testomony to the pliability of this area of the complicated, the highest of α6 of WzzB may be seen considerably bent in comparison with the upward and downward states of WzzE by 26° and 19°, respectively in direction of the middle of the complicated (Fig. 2b). Inside WzzE, this alternating association of the L4 loops leads to a refined rearrangement of its two flanking helices (α6 and α7): when within the upward dealing with place α6 helix is tilted approximated 9° outwards away from α7, and is barely elongated suggesting {that a} slight unraveling happens when L4 is within the downward place (Fig. 2c); and when the L4 loop is within the downward place α7 tilts barely inwards in direction of the middle of the periplasmic bell (Fig. 2c). Thus, as a consequence of the up-down nature of the L4 loops the complete octameric construction of WzzE incorporates a 13 Å opening on the prime of its periplasmic bell almost double the space in comparison with that of WzzB containing all downward L4 loops at 7.5 Å at their narrowest factors (Fig. 3a, b).
a Structural alignment of adjoining WzzE protomers containing an up (inexperienced) and down (cyan) L4 loop to a protomer of WzzB (PDB id 6RBG, faint white) with labels of the α-helices and loops mentioned within the textual content. In pink, α5 is lacking in WzzB. b Zoom of the L4 area exhibiting its dynamics. Left: within the upwards conformation and proper: within the downwards conformation aligned to the homologous area of WzzB. c Overlay of the 2 L4 loop conformation of WzzE.
a View wanting down from the highest of the periplasmic area of WzzE. The all-up and all-down variations of WzzE had been artificially generated by making use of C8 symmetry to a single subunit containing an upward and downward dealing with L4, respectively. b View wanting down from the highest of the periplasmic area of WzzB (PDB id 6RBG). c Zoom of the interplay between α6 and α7 of adjoining subunits of WzzE. High: the substitute all-down model. Backside: the naturally occurring alternating up-down model. d Overlay of the substitute all-down model of WzzE (cyan) to the naturally occurring all-down WzzB (PDB id 6RBG, salmon). High: view wanting down from the highest of the periplasmic domains. Backside: zoom of the interplay between α6 and α7 of adjoining subunits. In all panels, the pink bars symbolize clashes with a van der Waals distance ratio of lower than 0.89. Clashes between the α6 and α7 of adjoining subunits of WzzE will seemingly stop two adjoining WzzE downward dealing with subunits. These clashes are absent in WzzB as a consequence of a shortened α7, permitting adjoining downward subunits.
The alternating association of the L4 loops is a conserved, sturdy characteristic of WzzE
Regardless of intensive 2D and 3D classifications of the WzzE single-particle knowledge no different conformations apart from an alternating L4 could possibly be discovered; i.e., no WzzE complexes containing adjoining upward or adjoining downward dealing with L4 loops. To raised perceive why L4 has to alternate between adjoining WzzE protomers we generated synthetic all-up and all-down verisons of WzzE by making use of C8 symmetry to a single protomer containing an upward dealing with L4 and a single protomer containing a downward dealing with L4 (Fig. 3). The naturally occurring alternating WzzE has a conflict profile remarkably much like that of the WzzB (Fig. 3a, b) with solely two clashes (van der Waals distance ratio of lower than 0.89) between adjoining protomers detected. The all-up and all-down variations of WzzE alternatively, produced very completely different profiles. The all-up model incorporates no such clashes between adjoining protomers whereas the all-down model produced important clashes between α6 and α7 of adjoining protomers which might be absent within the alternating WzzE model. Though the all-down model of WzzE is strikingly much like WzzB when seen from the highest of the periplasmic bell with each having a diameter of 55 Å, the clashes between α6 and α7 of adjoining protomers are lacking in WzzB. This may be defined by a shortened α7 helix in comparison with WzzE (Fig. 3c, d and Supplementary Fig. 4). The shortening of α7 and thus the elimination of inflexible secondary construction components could clarify why WzzB can undertake down-down L4 preparations whereas WzzE can’t. Alternatively, why up-up variations of WzzE are usually not seen is just not instantly evident. Probably protomer-protomer stabilizing interactions are lacking on this conformation leading to an unstable complicated or L4 loop association. Thus, it’s doable that the alternating association strikes the proper steadiness of stability to permit for a dynamic complicated.
A ConSurf15,16 evaluation of WzzE revealed an identical sample of conserved residues as seen in WzzB7, with amongst others, patches of conserved residues flanking the L4 loop inside α7 and the highest of α6 (Fig. 4a), with the L4 loop itself containing primarily variable residues much like WzzB. Conserved patches of residues alongside α7 additional cement the significance of this area of the complicated and recommend the elongated α7 is a distinguishing characteristic of WzzE and sure the driving power behind the alternating nature of the L4 loops. The importance of those outcomes is additional supported by a earlier mutational examine13 by which strings of 5 amino acids had been inserted into this area of WzzB, leading to both the full lack of polysaccharide chain size regulation or the detection of solely brief polysaccharide chains.
a ConSurf evaluation of WzzE. Boxed: zoom of the L4 area. R267 is very conserved. b The interplay of R267 with the downward dealing with L4 with corresponding map density across the area of R267 (blue mesh). c Cartoon illustration of the complete octameric complicated of the R267A variant overlaid with the C4 symmetrized map (white) (left). Proper: Zoom of the L4 area and corresponding map density round A267 (prime) Backside: overlay with the native WzzE confirms a extremely comparable construction. R267A shows a small however noticeable 2 Å shift upwards beginning across the backside of α6 (pink line). d Cartoon illustration of the complete octameric complicated of the R267E variant overlaid with the C4 symmetrized map (white) (left). Proper: Zoom of the L4 area and corresponding map density round E267 (prime). Backside: overlay with the native WzzE confirms a extremely comparable construction.
The ConSurf evaluation additionally revealed an arginine inside α7 (R267) that interacts with the downward dealing with L4 (Fig. 4b) to be extremely conserved (Fig. 4a and Supplementary Fig. 5). To research how sturdy the alternating conformation of the L4 loops is, we changed this residue with an alanine and a glutamic acid in two constructs. Though the change of this amino acid is clearly seen within the cryo-EM density map, substitute had little impact on the general octameric construction of WzzE or the alternating association of the L4 loops (Fig. 4c, d and Supplementary Fig. 6). The R267A variant did, nonetheless, have a small, however noticeable 2 Å shift upwards of all the L4 area suggesting that this conserved residue might one way or the other assist stabilize this in any other case dynamic area of the complicated. Though there are solely small variations between the native WzzE and each R267 variants, these variants do underscore the character of the alternating loops in WzzE, as each buildings of the mutants had been solved independently utilizing reference-free ab-initio beginning volumes and clearly show the identical sample of alternating L4 loops of their unsymmetrized C1 maps because the native WzzE. The outcomes of those variant proteins thus recommend that the alternating conformation of the L4 loops is a strong characteristic ruled by world structural properties whereas the conservation of R267 could also be associated to substrate binding, as mutations to this area in WzzB resulted within the detection of solely brief size polysaccharide chains13.
The alternating L4 loops are dynamic and create a negatively charged potential sugar binding face alongside the inside of WzzE
To substantiate the dynamic character and alternating loop nature of this area in WzzE, a 3D variable evaluation (3DVA)17 was carried out on an unsymmetrized map centered on the highest of periplasmic bell (Supplementary Film 1). Regardless of the small measurement of this area of the complicated, which is probably going hampering any such evaluation, clear motion of the L4 loop and flanking α6 and α7 helices may be seen along with the easy fluctuation that’s sometimes noticed in inflexible buildings17. The evaluation reveals a transparent bending inward and downward of α6 in upward dealing with protomers, whereas the protomers with downward dealing with L4 loops, α6 seems to be concurrently bending barely backward and upwards, albeit a lot much less pronounced in comparison with the upward variations. Though the buried, extra hidden nature of the downward dealing with L4 loops could possibly be impeding the 3DVA, it might recommend that the downward dealing with L4 loops preserve their downward place whereas the upward L4s and flanking α6 and α7 helices are cell. Moreover, α7 of each protomers can be seen subtly adjusting to accommodate the motion of the adjoining α6 helices. The direct visualization of motion within the cryo-EM density additional verifies the dynamic, alternating loop nature of this area of WzzE. Furthermore, the back-and-forth motion of the helices might recommend a ratchet-like mechanism in polysaccharide elongation.
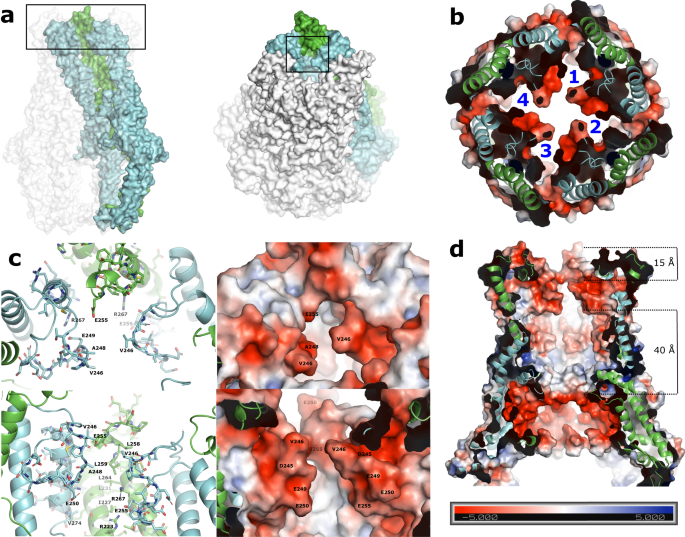
For the reason that L4 loops are important for manufacturing of lengthy polysaccharides10,13,14, however are positioned on the prime of the periplasmic area, too removed from the internal membrane to bodily work together with the polysaccharide polymerase WzyE, it has been urged that L4 should operate as a sugar-binding face for the rising polysaccaride10. This construction helps that concept and advances it by suggesting that three adjoining monomers encompassing the down-up-down conformations (Fig. 4a) do in actual fact create a really engaging potential sugar binding face (Fig. 5a–c). The created binding face is made up of the internal face of α6 (residues 223-239) in addition to most of the residues of the 2 downwardly oriented L4 loops on reverse sides. The center L4 loop is accommodated within the upward conformation, and apart from E255, is especially pointing away from the binding face. Along with primarily aliphatic residues this binding face incorporates an aspartic acid and a variety of glutamic acid residues, making a extremely adverse pocket (Fig. 5b, c). The alternating nature of the L4 loops create 4 binding faces per octameric complicated, and an electrostatic floor map of the complete WzzE (Fig. 5d and Supplementary Fig. 7a) reveals a further extremely negatively charged belt across the inside of the periplasmic bell, simply above the membrane interface that’s separated by 40 Å from the negatively charged binding floor created by the L4 loops (Fig. 4b). In between these two extremely adverse inside surfaces is a band of versatile loops (Supplementary Fig. 7b). There was many, seemingly contradictory, research suggesting that the rising LPS molecule can work together with both the inside or the outside of the periplasmic bell10,13,14,18,19,20. Though with out direct experimental proof, we can’t rule out the potential of the ECA chain rising on the skin of WzzE, nonetheless, with two inside extremely concentrated adverse bands and an inside band of versatile, extra neutrally charged loops in between, the construction introduced herein favors fashions that place the rising ECA molecule alongside the inside floor of the Wzz octamer.
a Left: Floor illustration of the octameric complicated. Three coloured adjoining protomers make up a possible binding face created by the positioning of the L4 loops, one up (inexperienced) surrounded by two down (cyan). The L4 area is boxed. Proper: tilted by 45° to show the binding pocket created by the L4 loops (boxed). b Electrostatic floor illustration exhibiting a extremely adverse binding face created by the positioning of the L4 loops. View wanting down from the highest of the periplasmic area sliced to show the 4 binding faces created by the positioning of the L4 loops per octameric complicated. c Zoom of the binding face labeled with its floor residues. Proper: electrostatic floor illustration exhibiting a extremely adverse binding face. d Electrostatic floor illustration of the octameric periplasmic area sliced to show the inside. Along with the adverse binding face of the L4 loops, the inside floor incorporates a further extremely adverse band simply above the membrane interface. A distance of 15 Å separates the highest of the upward L4 and the highest of the L4 binding face. The 2 adverse bands are separated by 40 Å.
A possible ratchet-like mechanism for polysaccharide elongation
As talked about, the alternating association of the L4 loops across the octameric bell would seemingly create 4 binding faces per complicated (Fig. 5b). Nevertheless, if the downward loops are shared between adjoining binding faces a most of two rising ECA molecules contained in the bell might occupy reverse dealing with binding faces at any given time because the downward L4 loops would in any other case be engaged with an ECA molecule and thus unavailable to the neighboring binding floor. This could possibly be according to studies of the polymerase Wzy forming dimers21,22, and a current Alphafold prediction of the polymerase binding on the inside face of Wzz23; thus, a polymerase dimer might probably span the transmembrane inside permitting the elongation of two ECA chains per octamer. This might additionally recommend that in actual fact, though this construction is octameric, a minimal down-up-down trimeric WzzE complicated would solely be required for correct chain size regulation of the ECA molecule. This may be according to features of the “clock mannequin” that was remarkably hypothesized 30 years in the past24, suggesting that the energetic type of Wzz is, in actual fact, a trimer. Moreover, crosslinking experiments that recommend that Wzz oligomerization is very dynamic in nature13,18,25,26 the place conflicting studies recommend trimeric, pentameric, hexameric, and octameric preparations of WzzB and WzzE7,8,9,10,12.
Lastly, to additional discover the potential of this putative binding face to accommodate sugar molecules, molecular docking was carried out utilizing a 2-repeating unit of the terminal finish of the negatively charged ECA molecule with a truncated octameric WzzE (residues 201–294) (Supplementary Fig. 8). The ensuing docking trials confirmed a transparent desire for the position of the sugar into this binding face, with solely 2 trials out of 32 being positioned exterior of this binding face, with remarkably, no sugars being positioned on the skin of the bell. Though these docking outcomes must be confirmed experimentally, they do current an surprising results of a negatively charged molecule docking to a negatively charged binding face. Although shocking, this might in actual fact be helpful for ECA development as it could create an unstable complicated between Wzz and the sugar chain permitting the ECA elongation course of to proceed. Any too sturdy binding would seemingly consequence within the elongation course of being halted. In reality, if this putative binding face may be confirmed experimentally, it could possibly be exploited with the event of particular positively charged inhibitors that would tightly bind to this negatively charged binding face and primarily block ECA elongation.
The alternating up-down L4 conformations (Supplementary Film 2) may instantly recommend that ECA polymerization might proceed by way of a ratchet-type mechanism. This could possibly be considerably analogous to the unidirectional transport mechanism of LPS molecules from the internal to the outer membrane revealed by current structural research on the Lpt system27,28. If a rachet-type mechanism29 is used for LPS transport, it stands to purpose it may be used for LPS biosynthesis. The putative binding faces created by the alternating L4 loops described right here might probably act in a concerted method with the negatively charged ECA molecules permitting them to ratchet upwards (probably one repeating unit at a time) by the dynamic L4 loops. Though the binding face is very adverse within the captured state described right here, the motion of L4 would seemingly alter its floor traits and thus its affinity to negatively charged ECA chain probably allowing a ratcheting sort mechanism to proceed. Moreover, the 3DVA might recommend that the way more secure downward dealing with L4 loops preserve their downward place to protect the potential binding face whereas solely the upward L4 ratchets up and down (Fig. 6). This may be according to the a lot increased decision and decrease B-factors of the downward L4 loops in comparison with the upward dealing with L4s (Supplementary Figs. 3, 6, and 7b). Moreover, because the conflict evaluation means that adjoining protomers can’t concurrently be within the downward orientation, the ratcheting of the upward L4 would seemingly be modulated by repulsion forces between neighboring α6 and α7 helices. Thus, like a ratchet, the upward L4s might solely go downward to some extent the place the repulsive forces from the neighboring α6 and α7 helices would power them upwards once more. Lastly, any ratcheting-type mechanism of the L4 loops might moreover be aided by the band of versatile, extra neutrally charged loops situated additional down the inside of the periplasmic bell. Though the mechanism of the polysaccharide chain elongation course of is at present unknown, the decided buildings and evaluation introduced right here present new insights that can promote new mechanistic concepts to evolve in direction of elucidating a mechanism of LPS polymerization and size regulation by the Wzy-dependent pathway typically, and the one containing WzzE particularly.
a Schematic mannequin of WzzE with alternating L4 loops. Strong black strains symbolize the place of the internal membrane. Inexperienced: subunits containing the upward oriented L4 loops. Blue: subunits containing the downward oriented L4 loops. b Zoom of the L4 loop area. The upward L4s (inexperienced) can solely go downward to some extent the place the repulsive forces from the neighboring subunits would power them upwards once more. The downward L4s stay comparatively secure, sustaining the binding face. Proper: view wanting down from the highest of the periplasmic area.