Typically, the properties of a molecule are predicted lengthy earlier than it’s synthesised. One such is diberyllocene. I first encountered a associated molecule, beryllocene itself, many moons in the past.[1] This was uncommon as a result of in contrast to the unique metallocenes, the metallic atom was not symmetrically disposed between the 2 cyclopentadienyl faces. Now diberyllocene is lastly reported through which changing one Be by Be-Be induces (in keeping with calculation, D2) symmetry[2]. I cannot repeat the superb evaluation of the wavefunction reported on this article, however confine myself to displaying two molecular orbitals which examplify its bonding.
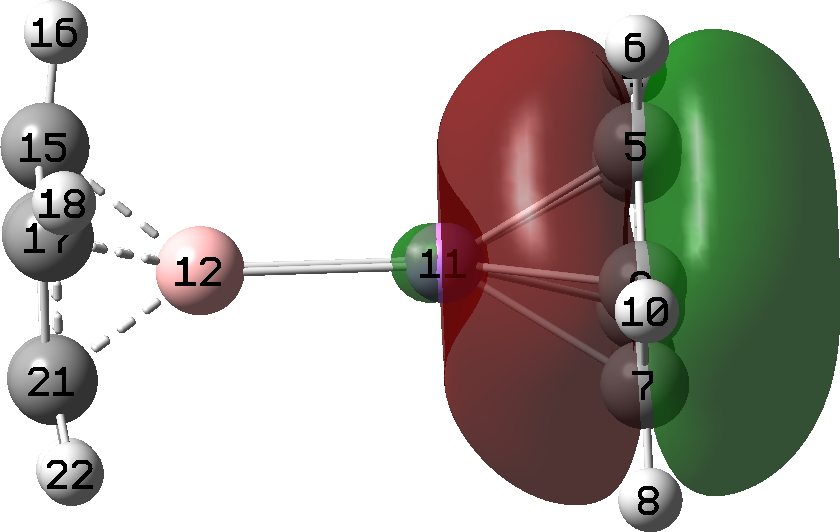
| Highest occupied molecular orbital |
|---|
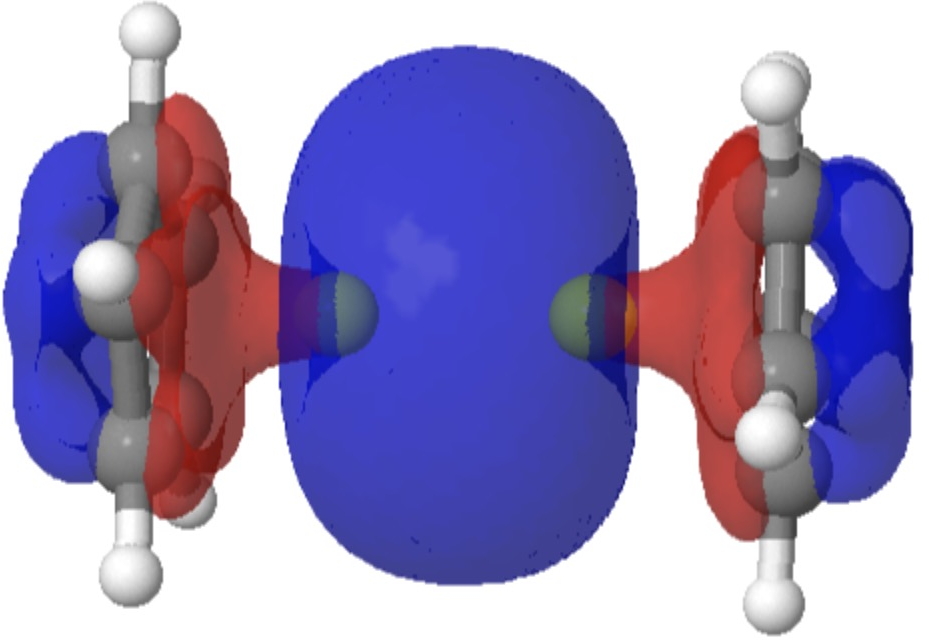 |
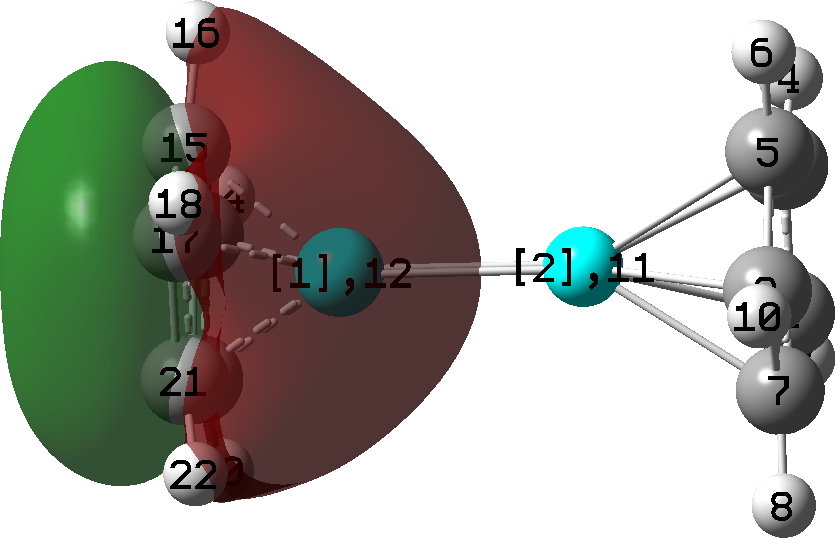
| Lowest occupied π-molecular orbital |
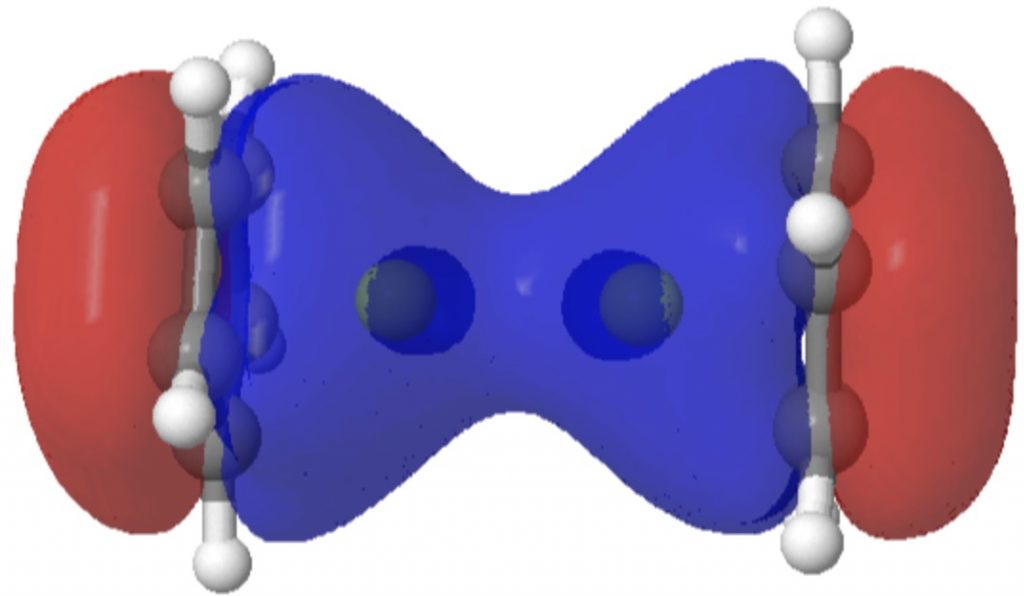 |
The HOMO (FAIR knowledge 10.14469/hpc/12702) basically reveals a Be-Be single bond, originating formally from the central Be22+ dication, balanced by the 2 cyclopentadienyl anion ligands. Click on on the photographs to see this orbital in 3D.
Oddly, an excited state of Be2 by itself really carries a Be=Be double bond, a property once more predicted a very long time in the past by idea. Probably the most secure π-MO in diberyllocene originates within the six electron fragrant cyclopentadienyl rings. By buying a share of six electrons from one Cp ring, and a share of the 2 electrons from the Be-Be bond, every Be atom achieves the octet of electrons recognized by generations of scholars. That is the form of molecule that might be taught in colleges at an early stage for instance the octet rule. And its good to know that straightforward new molecules illustrating this are nonetheless being found by chemists.
Since this began with experimental realisation of a predicted molecule, can I recommend as a brand new prediction, lithioborocene, through which a B and an Li exchange two Be atoms? Individually, lithiocenes, boracenes and Li-B bonds are recognized from crystal constructions. So its not a manner out prediction to mix these observations. Any pleasant artificial chemist up for the problem?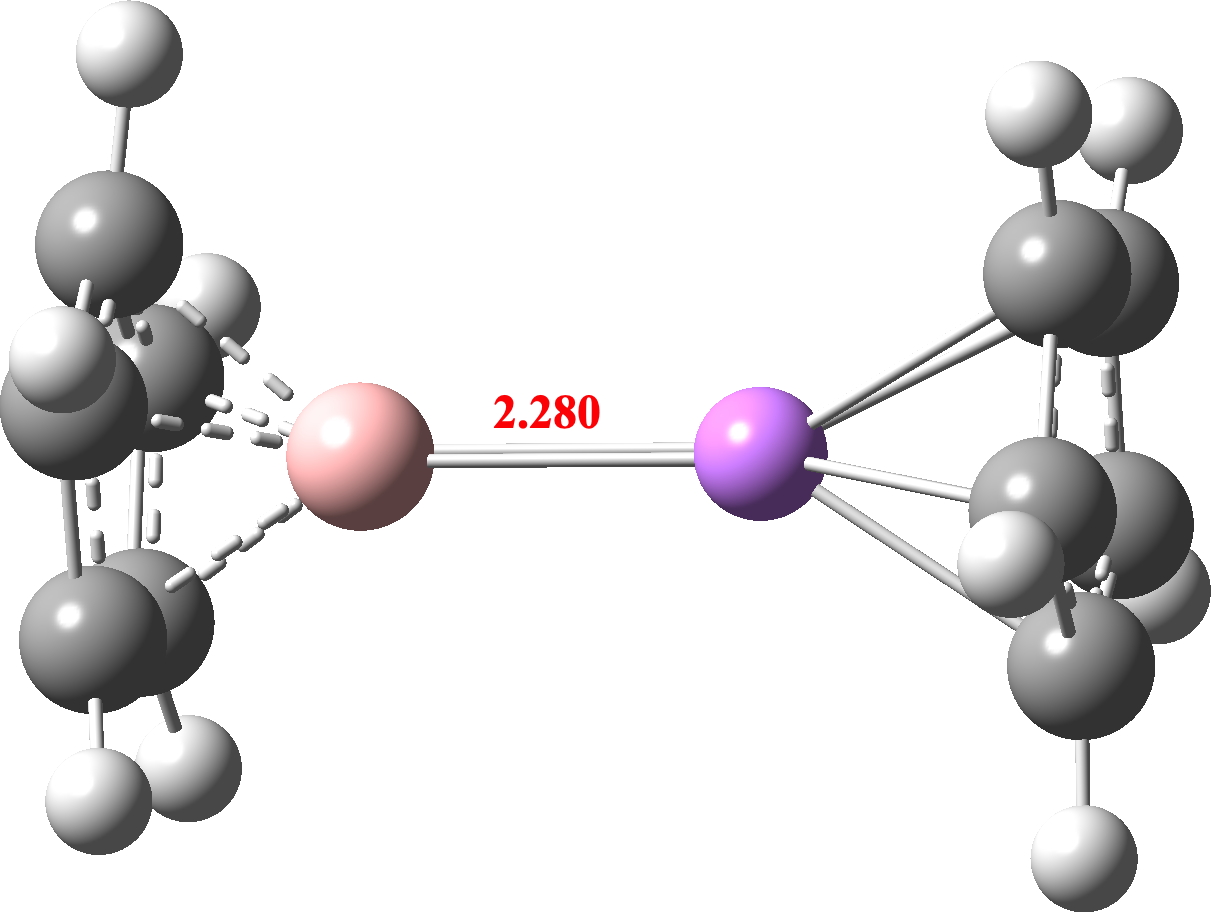
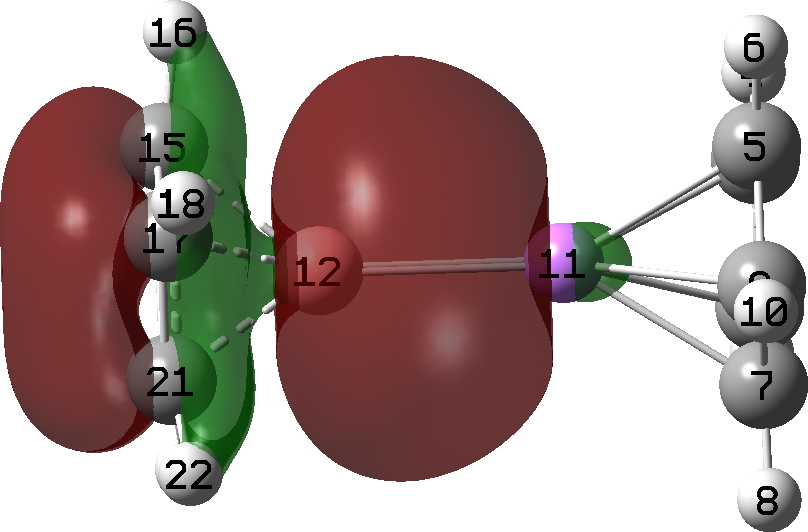
This submit has DOI: 10.14469/hpc/12704



