Engineering of Para by VSO
Fe3O4 magnetic nanoparticles (MNPs) have been synthesized by a solvothermal methodology utilizing sodium citrate as a modifier. The powder X-ray diffraction (pXRD) spectra confirmed the crystallinity of the obtained MNPs (Supplementary Fig. 1). For the reason that floor chemistry of MNPs could affect their stability in Para and their cytotoxicity towards Para, we employed sodium citrate and polymers, together with polyethyleneimine (PEI), polyethylene glycol (PEG), and polyacrylic acid (PAA), to change the MNPs, which have been then incubated with Para for two h. As indicated by the remaining Fe content material contained in the Para (Supplementary Fig. 2) and the cytotoxicity assay (Supplementary Fig. 3), sodium citrate-modified Fe3O4 (Fe3O4@sodium citrate) enabled environment friendly in vivo retention and confirmed much less cytotoxicity than the opposite modifiers. Thus, trisodium citrate dihydrate was added in the course of the synthesis of the MNPs earlier than antibody modification. The profitable coating of sodium citrate on the MNP floor was confirmed by analyzing the attribute peaks of the C–O stretching vibrations at 1396 cm−1, C = O stretching vibrations at 1597 cm−1 and O–H stretching vibrations at 3416 cm−1 after modification utilizing Fourier remodel infrared (FTIR) spectroscopy (Supplementary Fig. 4). The carboxy group from sodium citrate serves as a reactive website for antibody modification. The EV71 monoclonal antibody was connected onto the floor of MNPs utilizing N-ethyl-N′-(3-dimethylaminopropyl) carbodiimide (EDC)/N-hydroxysuccinimide (NHS) conjugation chemistry. Upon antibody conjugation, the zeta potential of MNPs@Ab shifted from −6 to −12 mV (Supplementary Fig. 5).
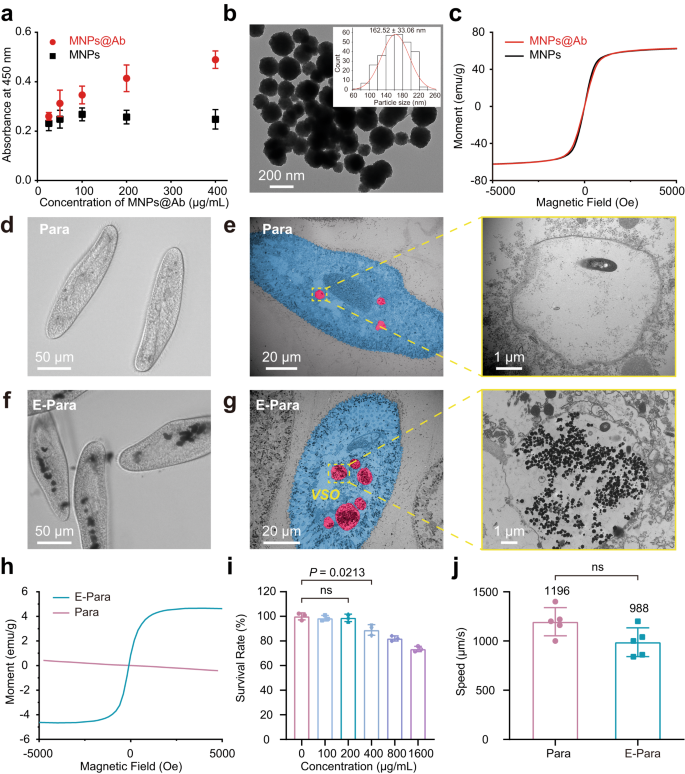
The virus binding affinity of MNPs@Ab alone was first examined by utilizing an enzyme-linked immunosorbent assay (ELISA) to substantiate that MNPs@Ab may acknowledge viruses. The elevated OD seen upon including MNPs@Ab urged MNPs@Ab preserved its binding affinity towards EV71 (Fig. 2a). Transmission electron microscopy (TEM) evaluation confirmed dimensions of MNPs@Ab with a diameter of ∼162 nm (Fig. 2b). Furthermore, the MNPs@Ab confirmed a saturation magnetization of ∼ 62 emu/g, which was similar to that of the MNPs management within the presence of antibody (Fig. 2c).
a ELISA of MNPs@Ab confirmed the antibody conjugation. The information are introduced because the imply ± sd (n = 3). b TEM picture of MNPs@Ab and measurement distribution of MNPs@Ab (inset). c Magnetic hysteresis loops of MNPs@Ab and MNPs. d, e Optical microscopy d and TEM photographs e of the meals vacuoles in pure Para. The highlighted vacuole space was enlarged. f, g Optical microscopy f and TEM photographs g of the meals vacuoles of E-Para. The highlighted VSO space was enlarged. h Magnetic hysteresis loops for pure Para and E-Para. i For fabrication of E-Para, Para have been cocultured with completely different concentrations of MNPs@Ab for two h. The obtained E-Para have been then cultured in medium with out feeding for an additional 24 h for toxicity analysis. The information are introduced because the imply ± sd (n = 3). j Speeds of pure Para and E-Para. The information are introduced because the imply ± sd (n = 3). In i, j, statistical significance was calculated by way of two-tailed Scholar’s t check. P < 0.05 was thought of vital. Ns not vital.
The in vivo VSO was engineered by feeding Para with MNPs@Ab (200 μg/mL)-containing modified Dryl’s answer (named KDS buffer, a phosphate buffer generally utilized in Paramecium research)35 for two hours at 25 °C. First, we used part distinction microscopy to look at the E-Para earlier than and after incubation with MNPs@Ab. As proven, pure Para confirmed clear vacuoles whereas E-Para displayed darkish and remoted vacuole-like buildings contained in the cells (Fig. 2d, f), which indicated the doorway of MNPs@Ab in the course of the feeding course of. TEM was then used to confirm the subcellular distribution of MNPs@Ab. The vacuoles of pure Para have been virtually empty attributable to hunger (Fig. 2e), whereas the entire vacuoles of E-Para have been crammed with giant quantities of MNPs@Ab (Fig. 2g). No vital distinction was noticed between the scale of the as-prepared MNPs@Ab and ingested MNPs@Ab in E-Para (in vivo) (Supplementary Fig. 6), indicating the steadiness of MNPs@Ab. Moreover, the magnetic hysteresis loop for E-Para confirmed superparamagnetic traits just like these of MNPs@Ab, whereas pure Para was diamagnetic (Fig. 2h). The intracellular MNPs@Ab have been then quantitatively evaluated utilizing inductively coupled plasma‒mass spectrometry (ICP‒MS), which confirmed 30.06 ± 2.44 μg Fe per 104 E-Para cells (Supplementary Fig. 7). These outcomes confirmed the efficient in vivo formation of VSOs inside Para (E-Para). Moreover, the MNPs@Ab-laden vacuoles have been secure in Para for no less than 24 h, exhibiting the steadiness of VSO in E-Para (Supplementary Fig. 8).
The impact of VSO on the organic properties of E-Para was additional examined. To evaluate the cytotoxicity of the implanted VSOs, we calculated the survival fee36 of Para after coincubation with a sequence focus of MNPs@Ab. The outcomes confirmed that MNPs@Ab exhibited minimized cytotoxicity to Para at concentrations as much as 200 μg/mL, manifesting acceptable biocompatibility (Fig. 2i). To estimate if the MNPs@Ab have an effect on the athletic efficiency of Para, we evaluated the motion pace of the E-Para. In contrast with that of pure Para (Supplementary Film 1), the pace of the E-Para decreased barely, which could be attributed to the elevated weight of the Para as a result of VSO implantation, however there was no vital distinction in line with the swimming pace (Fig. 2j) and course (Supplementary Film 2). These outcomes indicated that the E-Para remained viable after engineering.
Virus seize by E-Para
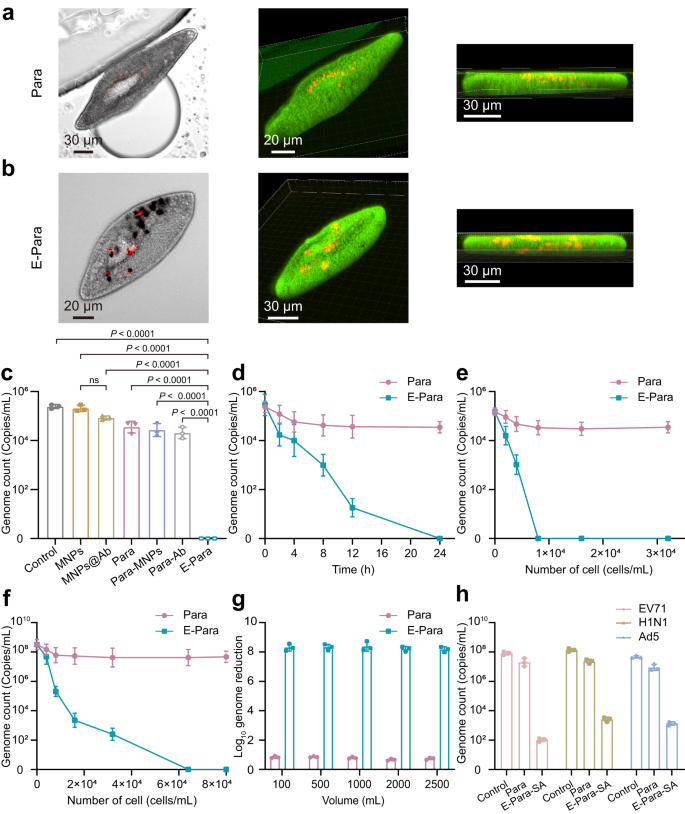
Then ingestion of virus was investigated by inserting E-Para or Para (8 × 103 cells) in 1 mL of EV71 (105 PFU/mL) for 4 hours at 25 °C. The Para and ingested viruses have been then noticed by utilizing confocal laser scanning microscopy (CLSM). Though purple dye-labeled EV71 was noticed in each teams, E-Para captured extra viruses than the pure Para, demonstrating that VSO enhanced the virus seize capability (Fig. 3a, b). Furthermore, we discovered that the purple indicators of the viruses have been utterly colocalized within the VSOs contained in the E-Para (Fig. 3b). Three-dimensional development photographs of the E-Para additionally satisfied that the viruses have been localized contained in the cells however not absorbed on the cell surfaces. We additionally used cross-sectional views of E-Para to substantiate that the EV71 was captured by MNPs@Ab (Supplementary Fig. 9).
a, b Section and CLSM photographs of Para a and E-Para b after capturing the EV71. In vivo EV71 was localized by merging the part and fluorescence photographs. Inexperienced represented CMFDA-labeled Para and purple represented AF555-labeled EV71. c To substantiate the virus seize capability of MNPs, MNPs@Ab, Para, Para modified with MNPs (Para-MNPs), Para fed with antibody (Para-Ab) and E-Para, the remaining EV71 within the water was examined after coculturing the above samples with EV71 for twenty-four h. The information are introduced because the imply ± sd (n = 3). Statistical significance was calculated by way of two-tailed Scholar’s t check. P < 0.05 was thought of vital. Ns not vital. d Time-dependent virus seize by Para and E-para. The information are introduced because the imply ± sd (n = 3). e Quantity of viral genome remaining in EV71-contaminated water (1.5 × 105 copies/mL) after therapy with completely different quantities of Para and E-Para for twenty-four h. The information are introduced because the imply ± sd (n = 3). f Quantity of viral genome remaining in EV71-contaminated water (3.2 × 108 copies/mL) after therapy with completely different quantities of Para and E-Para for twenty-four hours. The information are introduced because the imply ± sd (n = 3). g The of log10 reductions in viral genome ranges have been calculated after completely different volumes EV71 options have been handled with E-Para or Para (6.4 × 104 cells/mL). h For generic virus elimination, MNPs have been modified by sialic acid (SA), which concurrently grazed EV71, H1N1, and Ad5 from answer, reflecting the flexibility of this technique. The information are introduced because the imply ± sd (n = 3).
To find out whether or not the viral seize was depending on antibodies on the MNPs, we in contrast the virus seize efficiencies of MNPs, MNPs@Ab, Para engineered by MNPs with out antibody modification (Para-MNPs), or Para fed with antibody alone (Para-Ab) with that of E-Para by figuring out the viral genome remaining in suspension after 24 h of incubation. The presence of antibody on MNPs@Ab confirmed no vital enhancement of virus seize since MNPs is instable and have a tendency to combination in answer37, resulting in diminished virus binding affinity (Fig. 3c). In addition to, for Para-Ab, Para-MNPs, and native Para, their virus-capture capacity stays at low degree than that of E-para (Fig. 3c). For Para-Ab, the antibody was unable to succeed in a excessive native focus within the vacuoles. Thus, the ingested viruses weren’t effectively retained in vacuoles and may very well be excreted. In distinction, ingestion of MNPs@Ab in VSO resulted in a long-term retention and enrichment of antibody inside E-Para, which resulted in excessive virus seize effectivity. This outcome confirmed that the attachment of virus-specific antibody on MNPs considerably enabled the flexibility of VSO to seize viruses.
The virus-capturing efficiencies of E-para or Para over time have been then explored. For Para, 8 to 24 h of seize led to solely a 0.8 log10 discount within the quantity of viral genome. In distinction, the VSOs of E-Para drastically improved the seize effectivity over time and finally led to an entire scavenge of viral genome after 24 h (Fig. 3d). The remaining viruses within the supernatant have been additionally validated utilizing oblique immunofluorescence evaluation (IFA). These outcomes confirmed that variety of contaminated rhabdomyosarcoma (RD) cells was diminished after therapy with E-Para (Supplementary Fig. 10), which was according to the RT‒qPCR outcomes, revealing that E-Para captured the virus extra successfully than Para. These outcomes confirmed that VSO performs vital roles within the virus seize course of. The present problem for waterborne illness management is that the small sizes and really low concentrations in environmental water make virus clearance with standard filter units extraordinarily tough. Nevertheless, we discovered that E-para confirmed excessive efficacy for capturing viruses with smaller sizes (roughly 30 nm) and with decrease focus in an efficient and environmentally pleasant method with out the necessity for further units.
The dosage-dependent virus scavenging conduct of E-Para confirmed that EV71 at a focus of 1.5 × 105 copies/mL was utterly eliminated by 8 × 103 cells/mL of E-Para inside 24 h after incubation (Fig. 3e). The minimal focus wanted to clear these viruses was 8 × 103 cells/mL of E-Para (Fig. 3e). Of be aware, when the virus focus was elevated to three.2 × 108 copies/mL, no less than 6.4 × 104 cells/mL E-Para have been wanted to utterly clear these viruses, indicating dosage-dependent virus elimination (Fig. 3f). In distinction, a rise within the degree of native Para didn’t considerably enhance virus elimination (Fig. 3e, f), which was according to earlier outcomes21. The infectivity of the EV71-contaminated answer handled by E-Para was additionally measured with a plaque-forming assay (Supplementary Fig. 11). Moreover, the feasibility of VSO-based virus seize was decided by rising the volumes of the EV71-containing options. With will increase within the water quantity, the E-Para therapy resulted in an 8 log10 discount within the viral genome, exceeding the WHO customary (4 log10 discount worth) (Fig. 3g)38. Furthermore, the elimination efficacy confirmed no apparent lower when the amount was raised to 2500 mL, indicating preferable processing capability in giant volumes of water.
Antibody-based virus-specific seize is just not generic to different kinds of waterborne viruses. In case of purifying water of the broad number of waterborne viruses, equipping the E-Para with VSO that targets several types of potential viruses can also be required. Due to this fact, we modified the MNPs with sialic acid (SA), a monosaccharide and a key part for receptor attachment to viruses akin to enterovirus, influenza, and adenovirus39,40,41, to confirm the flexibility of the VSO-based technique. After modification of SA, the zeta potential of the MNPs@SA shifted from 26.6 to −9.1 mV (Supplementary Fig. 12a). The SA-modified MNPs (MNPs@SA) exhibited the attribute FTIR peaks for N–H stretching vibrations at 3385 cm−1, C = O stretching vibrations at 1617 cm−1, N–H bending vibrations at 1541 cm−1, C–O–C uneven stretching vibrations at 1278 cm−1 and C–O–C symmetric stretching vibrations at 1068 cm−1 (Supplementary Fig. 12b).
Para was engineered by MNPs@SA in the identical approach as E-Para and was named E-Para-SA. The E-Para-SA was used to deal with an answer containing EV71 (8.2 × 107 copies/mL), H1N1 (1.4 × 108 copies/mL) and Ad5 (4.6 × 107 copies/mL). As a result of attachment of the viruses to glycans of the sialic acid, the log10-reduction ranges for EV71, H1N1 and Ad5 by E-Para-SA have been 6, 5, and 5 respectively (Fig. 3h), indicating considerably greater virus-capturing effectivity than native Para. The elimination efficiencies for the three viruses reached 99.99%, which have been greater than 4 log10 copies/mL. These information indicated generic virus-capturing capacity, suggesting the flexibility of VSO will be tailor-made by rational design of practical teams on the MNPs.
Mechanism of virus seize by E-Para
How does MNPs@Ab-containing VSOs seize viruses? CLSM photographs of virus seize by E-Para confirmed the co-localization of viruses (purple) and MNPs@Ab (black in part picture) in the identical vacuoles of E-Para (Fig. 3b). This outcome verified the newly ingested viruses entered the pre-existing VSOs. To research how meals move from new vacuoles to VSO of E-Para, we incubated E-Para (Supplementary Fig. 13a, b) with Escherichia coli (E.coli), and noticed the formation of vacuoles contained each micro organism and MNPs@Ab on the identical time. E.coli have been ingested by E-Para by cytostome (Supplementary Fig. 13c) to type a brand new meals vacuole (FV) marked in blue (Supplementary Fig. 13d). We discovered E.coli-containing vacuoles fused with MNPs@Ab-containing VSO, indicating the meals passage by a vacuole’s fusion pathway (Supplementary Fig. 13e–h). These outcomes confirmed the virus seize by VSO is predicated on the meals passage of Para, by which virus-containing vacuoles fused with circulating VSOs in vivo. After getting into the VSO, the viruses have been captured by antibodies in VSO (Fig. 3c). If antibody is just not connected on MNPs, they have been unable to be entrapped and concentrated inside vacuoles of Para for very long time, leading to low degree of virus seize (Fig. 3c). In distinction, the long-term retention and enrichment of MNPs@Ab in VSO resulted in a excessive native antibody focus in vacuoles (Supplementary Fig. 8), which is useful for virus seize and entrapment by antibodies inside VSO, thus enhancing the virus-capture effectivity of E-Para (Fig. 3c).
Inactivation of viruses by E-Para
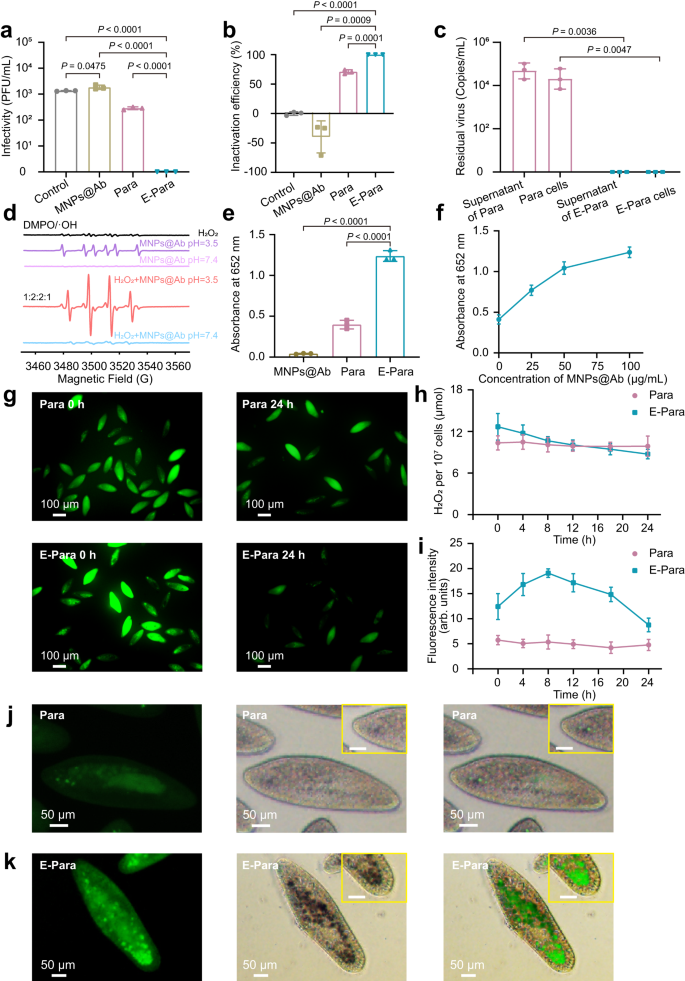
Virus inactivation by E-Para was assessed by analyzing the infectivity of E-Para-captured viruses utilizing plaque-forming assays. After EV71 was captured for twenty-four h, the viruses inside Para and E-Para have been launched by cell lysis therapies after which used to contaminate RD cells to find out the remaining infectivity. As a management, cell lysis therapy with the SDS lysis buffer confirmed little impact on viral infectivity (Supplementary Fig. 14). Of be aware, the viruses ingested by E-Para utterly misplaced their infectivity (Fig. 4a), which indicated that the E-Para not solely captured the viruses but additionally inactivated them. In distinction, the titer of viruses captured by pure Para have been diminished by lower than 1 log10 PFU/mL, suggesting that the viruses weren’t utterly inactivated by pure Para (Fig. 4a). Moreover, MNPs@Ab alone had no virucidal impact in water (Fig. 4a). The inactivation of ingested viruses by E-Para approached 100%, whereas pure Para and MNPs@Ab confirmed 70.87% and no inactivation respectively (Fig. 4b). Taken collectively, these information point out that the viruses remained infectious contained in the vacuoles of native Para or after therapy with MNPs@Ab, whereas the VSOs inside E-Para utterly inactivated the infested viruses, implying a synergetic deactivation impact of MNPs@Ab in vacuoles. Para shielded the viruses and viral genomes contained in the vacuoles, which remained infectious. We then investigated whether or not the E-Para or Para utterly inactivated the ingested virus after incubation with EV71 for twenty-four h. For pure Para, the ingested virus remained infective and the viral genome was detectable, indicating the potential danger for utilizing pure for virus seize. Nonetheless, for E-Para, no residual viral genome was detected in VSO (Fig. 4c). These outcomes indicated that E-Para successfully scavenged the ingested viruses contained in the VSOs.
a Intra-Para EV71 titer detected with the plaque-forming assay. b Inactivation efficiencies of MNPs@Ab, Para and E-Para. c Viral genome remaining inside E-Para or Para cells after therapy. d EPR spectra of MNPs@Ab at completely different pH values. e Comparability of the catalytic capacities of MNPs@Ab, pure Para and E-Para to TMB. f The dose-dependent catalytic capability of E-Para. g Fluorescence photographs of Para and E-Para stained with ROSGreenTM (a H2O2 probe with inexperienced fluorescent). h H2O2 contents in Para and E-Para. i Fluorescence depth of •OH measured by ImageJ. The information are introduced because the imply ± sd (n = 3). j, ok Fluorescence photographs of Para j and E-Para ok stained with HPF (a •OH probe with inexperienced fluorescent). The photographs within the yellow packing containers are partially enlarged (Bar, 20 μm). Statistical comparisons have been made utilizing both two-tailed Scholar’s t check (a, b, e) or two-way analyses of variance (ANOVA) with Tukey’s multiple-comparison check c. P < 0.05 was thought of vital. Ns not vital.
Hydroxyl radical-based virus deactivation mechanism
Since ferric oxide has peroxidase-like exercise in acidic pH environments, the MNPs@Ab within the VSOs is able to producing hydroxyl radical (•OH) by catalyzing hydrogen peroxide (H2O2) by Fenton-like response34. •OH can inactivate viruses as a result of it reacts with virtually all kinds of biomolecules, akin to lipids and nucleotides42,43. To research the peroxidase-like exercise of MNPs@Ab in vitro, electron paramagnetic resonance (EPR) spectroscopy was used. At pH 3.5, MNPs@Ab displayed stronger dose-dependent EPR indicators (1:2:2:1) with DMPO/•OH (Supplementary Fig. 15) within the presence of H2O2 than it did with out added H2O2 (Fig. 4d). Nonetheless, no •OH sign was detected at pH 7.4 for MNPs@Ab or the combination of MNPs@Ab and H2O2 (Fig. 4d). Due to this fact, in acidic options containing H2O2, MNPs@Ab generated extremely reactive •OH.
With the intention to confirm the antiviral capacity of MNPs@Ab-induced •OH in vitro, we incubated MNPs@Ab (200 μg/mL) with EV71 within the presence of H2O2 (1 mM) at completely different pH for twenty-four h and examined remained titer of EV71 by plaque assay. In acidic options containing H2O2, infectivity of EV71 was successfully diminished by MNPs@Ab (Supplementary Fig. 16), as a result of era of extremely reactive •OH (Fig. 4d and Supplementary Fig. 15). Quite the opposite, beneath pH of 6, 7, and eight, the MNPs@Ab was unable to inactivate EV71.
We additionally examined the peroxidase-like exercise of MNPs@Ab contained in the VSOs in vivo. 3,3,5,5-Tetramethylbenzidine (TMB), a substrate of peroxidase, reacts with ferric oxide within the presence of H2O2 beneath acidic situations to develop a blue coloration with a most absorbance at 652 nm33. We subsequently added TMB to KDS buffer containing E-Para to search for the blue product. As anticipated, each Para and E-Para cells produced a blue catalysate in vivo (Supplementary Fig. 17), whereas the absorbance at 652 nm for TMB-treated E-para was evidently stronger than that of pure Para (Fig. 4e), indicating a bolstered catalytic response occurred inside E-Para. Notably, the enzyme exercise elevated because the focus of MNPs@Ab elevated, confirming that the catalytic impact was certainly associated to MNPs@Ab within the VSOs (Fig. 4f).
To examine the presence of H2O2 inside Para, ROSGreenTM, a particular H2O2 probe was used that emits inexperienced fluorescence upon contact with H2O2. Each Para and E-Para exhibited the presence of intracellular H2O2 (Fig. 4g), which was primarily derived from lysosomes or peroxisomes within the cytoplasm44. As a result of presence of MNPs@Ab, the H2O2 ranges within the VSOs elevated extra quickly than they did with Para inside the first 8 h, after which decreased sooner than they did with Para, suggesting accelerated H2O2 consumption (Fig. 4h).
There have been in depth research illustrating that the meals vacuole of Para undergoes a interval with acidic pH30,32,45, which, along with the H2O2 contained in the vacuole, creates favorable situations for the response between Fe (II) and H2O2 that yields •OH. We used 3′-(p-hydroxyphenyl) fluorescein (HPF) to trace the formation of •OH. Curiously, extra aggregated inexperienced fluorescent vacuoles have been noticed in as-prepared E-Para (Fig. 4k) than in pure Para (Fig. 4j) at 0 h, verifying the speedy manufacturing of •OH contained in the VSOs. The •OH manufacturing was then considerably enhanced in E-Para 8 hours submit VSO engineering, indicating manufacturing of enormous quantities of •OH within the VSOs (Fig. 4i). Thereafter, the quantity of •OH started to say no, most likely as a result of consumption of •OH throughout virus inactivation course of. The colocalization of intracellular MNPs@Ab and the •OH fluorescence sign confirmed the essential function of MNPs@Ab within the era of •OH contained in the vacuoles (Fig. 4k).
These phenomena indicated that the VSOs in E-Para resulted in a constantly greater degree of •OH than native Para, resulting in redox injury to the ingested virus. Collectively, the VSOs utilized synergistic interaction between MNPs@Ab and the vacuole surroundings to comprehend sustained manufacturing of •OH, which enabled environment friendly virus inactivation.
Recyclability of E-para
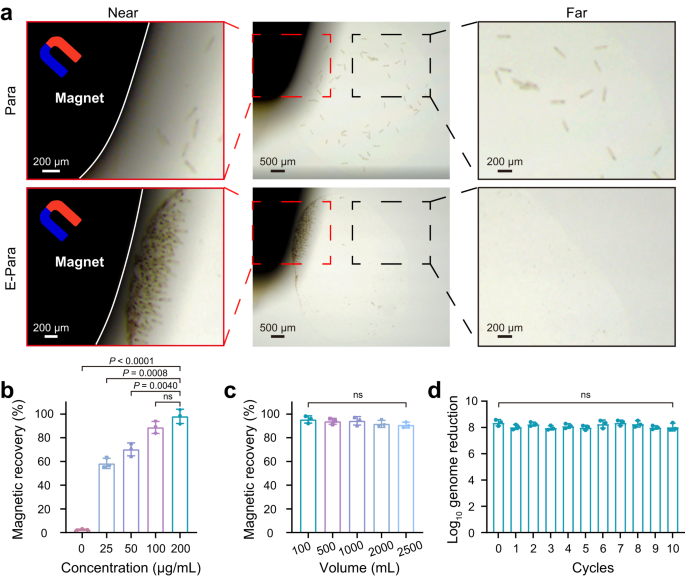
Within the case of biosecurity points attributable to virus-captured E-Para within the water, it’s important to recuperate the used E-Para after therapy. Nevertheless, Para are tough to gather by standard assortment strategies attributable to their excellent motility. The VSO-implanted E-Para was simply recovered from water answer by an exterior magnet (Fig. 5a) and returned to free motion after the magnet was eliminated (Supplementary Film 3), confirming that magnetic restoration had no vital impact on the exercise of E-Para. As well as, we killed E-Para by 4% paraformaldehyde and located that the killed E-Para confirmed superparamagnetic attribute just like that of MNPs@Ab, indicating that the killed E-Para can also be recyclable due to VSO (Fig. 2h). The magnetic restoration effectivity of E-Para was associated to the focus of MNPs@Ab in VSO (Fig. 5b). As well as, magnetic restoration of E-Para from varied volumes of answer was unaffected by the elevated quantity of water, indicating the provision of E-para elimination with out the necessity for further therapy of the answer (Fig. 5c).
a Restoration of Para and E-Para with a magnet. The photographs have been collected with a stereomicroscope. b Impact of included MNPs@Ab focus on magnetic restoration of the E-Para. The information are introduced because the imply ± sd (n = 3). c Impact of answer quantity on the magnetic restoration of E-Para. The information are introduced because the imply ± sd (n = 3). d Reuse of E-Para. The information are introduced because the imply ± sd (n = 3). In b, c and d, statistical significance was calculated by way of two-tailed Scholar’s t check. P < 0.05 was thought of vital. Ns not vital.
Nevertheless, the magnetism variation of E-Para over time exhibited a slight lower within the saturation magnetization from 3.4 to 1.7 emu/g inside 24 h (Supplementary Fig. 18a). The Fe content material within the E-Para stored declining inside 72 h (Supplementary Fig. 19a), which led to the discount of saturation magnetization. Nevertheless, discount of MNPs had negligible influence on the magnetic restoration fee of E-Para inside 24 h (Supplementary Fig. 18b). As well as, when E-Para was cultured in hunger medium with time, their viability started to say no after 24 h (Supplementary Fig. 19b), indicating long-term toxicity of MNPs@Ab on Para. Proliferation of E-Para needs to be additionally restricted by utilizing hunger medium to keep away from the discount of VSO attributable to the division of E-Para (Supplementary Fig. 19c). Collectively, to keep away from additional lack of recyclability, the virus seize time needs to be restricted to 24 h.
The recycled E-Para have been then cultured in development medium supplemented with E.coli to alongside the proliferation of Para (Supplementary Fig. 19d). To keep away from the lower of MNPs@Ab in particular person cells (Supplementary Fig. 19c), proliferated Para have been re-engineered with MNPs@Ab to revive the VSOs earlier than reuse. The reactivated E-Para nonetheless resulted in an 8 log10 discount of viral genome ranges after ten cycles (Fig. 5d), indicating the feasibility of recycling and reusing this method. These outcomes present that the magnetic VSOs enabled environment friendly restoration of E-Para with a magnetic discipline, which facilitated reuse and prevented the chance of an infection, thus guaranteeing the environmental friendliness and biosafety of this technique.